
The true molecule consists of only ONE arrangement of nitrogen, oxygen and electrons. The NO 2 shows two nitrogen-oxygen bonds that are stable and both consistent with being the equivalent of one and one-half bonds. And as the right-side moves in, the left-side moves out. Since double bonds are shorter than singles, the O would pump in and out relative to the N. The it goes back to single, then double, single, double.
#Explain resonance in chemistry movie
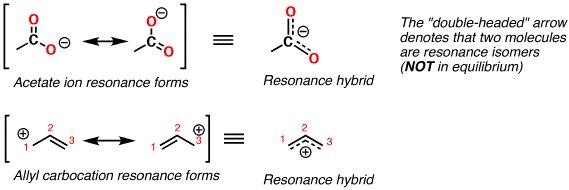
Like if you were to make a movie of, say, the right-side nitrogen-oxygen bond. What they do is they think that the actual molecule "flips" between the two structures (or "cycles through 3 or more structures). However, there is a big misconception that some people (hey, lots of people) get. OK, so you understand that the Lewis structures for NO 2, for example, don't really exist. It is for this practical reason that we find it convenient to speak of the resonance of molecules among several electronic structures." By using valence-bond structures as the basis for discussion, however, with the aid of the concept of resonance, we are able to account for the properties of the molecule in terms of those of other molecules in a straightforward and simple way. that the molecule cannot be satisfactorily represented by any single valence-bond structure and abandon the effort to correlate its structure and properties with those of other molecules. The two structures above are merely descriptive aids and, in fact, never exist. The real molecule acts as if it had one and one-half bonds between each of the two structures. The real molecule that exists in nature is a "resonance hybrid" between the two. Neither one of those two structures really does exist. Let your eye go back and forth between the two for a few seconds. Go back and stare at the the two NO 2 structures. Now we come to some tough parts of resonance. I will try and highlight this when I do CO and CO 2. When this happens, the true structure is a blend of all the different possible structures.Īlso, understand that the different structures contribute differently to the final structure. What is resonance? Resonance happens when more than one valid Lewis dot-diagram (or what Pauling calls a valence-bond structure) can be written for a molecule or ion. In the answer, I used RNO 2, where the R stands for Cl or F. However, I've put the answer in a different file, so you could try and figure it out on your own, if you wanted to. Let's try a compound that has the nitro-group attached, such as ClNO 2 or FNO 2. Once again, note that both structures are completely within the rules. Here is another example, using the molecule NO 2: Both structures obey all the rules and there is NOTHING to rule out one structure in favor of the other. Notice how the double bond can be shown attached to either oxygen. The acetate anion is an example (Replace R with -CH 3 to get acetate): we are now ready to begin the discussion of the structure of molecules to which a single valence-bond formula cannot be assigned." The last portion of the first sentence of "The Nature of the Chemical Bond" reads: Starting around 1930, Linus Pauling developed what today is called "resonance theory," the currently accepted way to explain the bonding in these substances. These substances must be described in terms of "intermediate" structures, possessing non-integral bonds such as one and one-half bonds or one and one-third bonds.īeginning in the 1920s, the first modern attempts to explain these structures started. This was known even back to the early beginnings of structural chemistry in the mid-1850s. There are a number of compounds and polyatomic ions that cannot be written using one single structure.


 0 kommentar(er)
0 kommentar(er)
