

In ethane, one carbon atom is bonded to four other atoms. In other words, a carbon atom can be bonded in four places.
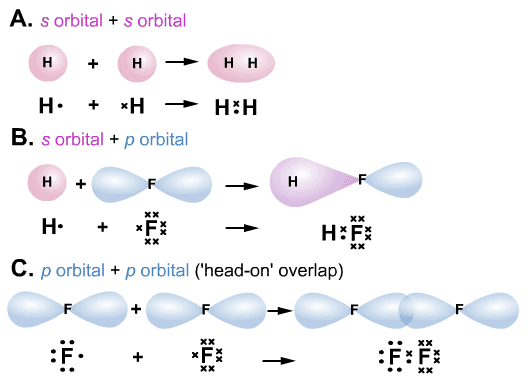
If we focus on the single-bonded carbon atom, the carbon atom has one s-orbital and three p-orbitals, which means that there are four hands. These orbitals are called s orbital and p orbital. An atom’s hand refers to its electron orbitals.Įach atom or molecule has orbitals. When atoms bond, they need to give their own hand. The sp3 Hybrid Orbitals Have Four Hands in s and p Orbitals There are two types of bonds between molecules: σ-bond and π-bond, and we must clearly distinguish between σ-bond and π-bond. When molecules are bonded, they are not bonded in this simple way, but actually bonded in a special way. As long as you have this image in your mind, you will not be able to understand the sigma and pi bonds in chemistry. What image do you have in mind when you understand the double bond of a compound? The majority of people who have taught high school chemistry, including when assembling a model of a molecule, have the following image of a bond.įorget about this molecular image. We Should Forget the Image of Double Bonds in High School Chemistry 4 Learn About the Types of Covalent Bonds and the Differences Between Them.3.3 Not All pi Bonds, Such As Benzene Rings and Carbon Dioxide, Are Weak Bonds.3.2 Highly Reactive Double- and Triple-Bonded of π-Bonds: Examples of Ethylene and Acetylene.3.1 Double and Triple Bonds Involve pi Bonds.3 π-Bonds (pi-Bonds) Are loosely coupled to the Bond Axis.2.2 Single σ-Bond Can Be Rotated: the Example of Ethane.2.1 σ-Bond (Sigma Bond) Forms a Covalent Bond and the Bond Energy Is High.2 The sp3 Hybrid Orbitals Have Four Hands in s and p Orbitals.1 We Should Forget the Image of Double Bonds in High School Chemistry.


 0 kommentar(er)
0 kommentar(er)
